Endocrine Hyperfunctioning
Hyperfunction of endocrine glands may result from overstimulation by the pituitary but is most commonly due to hyperplasia or neoplasia of the gland itself. In some cases, cancers from other tissues can produce hormones (ectopic hormone production). Hormone excess also can result from exogenous hormone administration. In some cases, patients take hormones without telling the physician (factitious disease). Tissue hypersensitivity to hormones can occur. Antibodies can stimulate peripheral endocrine glands, as occurs in hyperthyroidism of Graves disease. Disruption of a peripheral endocrine gland can rapidly release stored hormone (eg, thyroid hormone release in subacute thyroiditis). Enzyme defects in the synthesis of a peripheral endocrine hormone can result in overproduction of hormones proximal to the block. Finally, overproduction of a hormone can occur as an appropriate response to a disease state.
Endocrine Hypofunctioning
Hypofunction of an endocrine gland can result from understimulation by the pituitary. Hypofunction originating within the peripheral gland itself can result from congenital or acquired disorders (including autoimmune disorders, tumors, infections, vascular disorders, and toxins). Genetic disorders causing hypofunction can result from deletion of a gene or by production of an abnormal hormone. A decrease in hormone production by the peripheral endocrine gland with a resulting increase in production of pituitary regulating hormone can lead to peripheral endocrine gland hyperplasia. For example, if synthesis of thyroid hormone is defective, thyroid-stimulating hormone (TSH) is produced in excessive amounts, causing goiter.
Most hormones require conversion to an active form after secretion from the peripheral endocrine gland. Certain disorders can block this step (eg, renal disease can inhibit production of the active form of vitamin D). Antibodies to the circulating hormone or its receptor can block the ability of the hormone to bind to its receptor. Disease or drugs can cause increased rate of clearance of hormones. Circulating substances may also block the function of hormones. Abnormalities of the receptor or elsewhere in the peripheral endocrine tissue can also cause hypofunction.
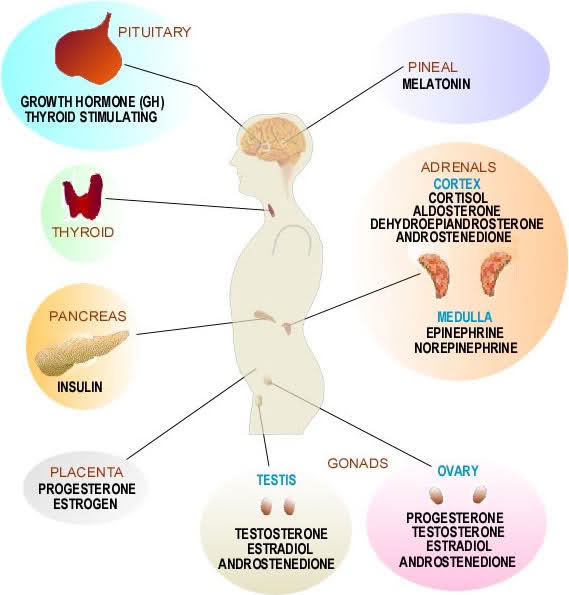
Aging and Endocrinology
Hormones undergo many changes as a person ages.
- Most hormone levels decrease.
- Some hormone levels remain normal, including TSH, ACTH (basal), thyroxine, cortisol (basal), 1,25-dihydroxycholecalciferol, insulin (sometimes increases), and estradiol (in men).
- Some hormone levels increase.
Hormones that increase, including ACTH (increased response to corticotropin-releasing hormone), follicle-stimulating hormone, sex-hormone binding globulin, and activin (in men), gonadotropins (in women), epinephrine (in the oldest old), parathyroid hormone,norepinephrine, cholecystokinin, vasoactive intestinal peptide, vasopressin (also loss of circadian rhythm), and atrial natriuretic factor, are associated with either receptor defects or postreceptor defects, resulting in hypofunction.
Many age-related changes are similar to those in patients with hormone deficiency, leading to the hypothesis of a hormonal fountain of youth (ie, speculation that some changes associated with aging can be reversed by the replacement of one or more deficient hormones). Some evidence suggests that replacing certain hormones in the elderly can improve functional outcomes (eg, muscle strength, bone mineral density), but little evidence exists regarding effects on mortality. In some cases, replacing hormones may be harmful, as in estrogen replacement in most older women.
A competing theory is that the age-related decline in hormone levels represents a protective slowing down of cellular metabolism. This concept is based on the rate of living theory of aging (ie, the faster the metabolic rate of an organism, the quicker it dies). This concept is seemingly supported by studies on the effects of dietary restriction. Restriction decreases levels of hormones that stimulate metabolism, thereby slowing metabolic rate; restriction also prolongs life in rodents.
Specific age-related hormone decreases
Dehydroepiandrosterone (DHEA) and its sulfate levels decline dramatically with age. Despite optimism for the role of DHEA supplementation in older people, most controlled trials failed to show any major benefits.
Pregnenolone is the precursor of all known steroid hormones. As with DHEA, its levels decline with age. Studies in the 1940s showed its safety and benefits in people with arthritis, but additional studies failed to show any beneficial effects on memory and muscle strength.
Levels of GH and its peripheral endocrine hormone (IGF-1) decline with age. GH replacement in older people sometimes increases muscle mass but does not increase muscle strength (although it may in malnourished people). Adverse effects (eg, carpal tunnel syndrome, arthralgias, water retention) are very common. GH may have a role in the short-term treatment of some undernourished older patients, but in critically ill undernourished patients, GH increases mortality. Secretagogues that stimulate GH production in a more physiologic pattern may improve benefit and decrease risk.
Levels of melatonin, a hormone produced by the pineal gland, also decline with aging. This decline may play an important role in the loss of circadian rhythms with aging.